Blood clotting complications after COVID-19 vaccines and COVID-19 infection: Paradoxes, similarities and health risks
There has been, and there still remains deep concern and confusion regarding the occurrence of blood clotting linked to COVID-19 vaccines, in particular, the AstraZeneca and Johnson & Johnson Janssen vaccines, which have been linked to a small number of fatalities. Although the incidence of such complications is extremely low, these events have fuelled the thriving scepticism which has been present since the beginning of the world-wide vaccination campaign.

In order to analyse the causality of such thrombotic events, major governmental institutions, first of all, the European Medicines Agency (EMA), have decided to temporarily suspend the use of AstraZeneca and the mono dose vaccine generated by Johnson & Johnson Janssen.
Let’s begin by understanding the incidence of this phenomenon, its definition in medical terms, the differences/commonalities of COVID-19 infection and COVID-19 vaccine-related blood clotting, and whether or not halting the use of these vaccines is actually justified after they have been inoculated in millions of individuals globally.
The AstraZeneca vaccine: composition and post-injection side effects
COVID-19 Vaccine AstraZeneca has been generated for preventing coronavirus disease 2019 (COVID-19) in people aged 18 years and older. COVID-19 is caused by SARS-CoV-2 virus. The vaccine has been authorised by the World Health Organization and the EU, but is not yet approved in the US. Unlike the Pfizer and Johnson & Johnson Janssen vaccines, the AstraZeneca vaccine is made up of an incompetent (non-infectious) chimpanzee adenovirus used as a vector/shuttle to deliver the gene (DNA) encoding the surface protein of SARS-CoV-2, also known as the “Spike” protein into our body. The spike protein is the common target for many COVID-19 vaccines.
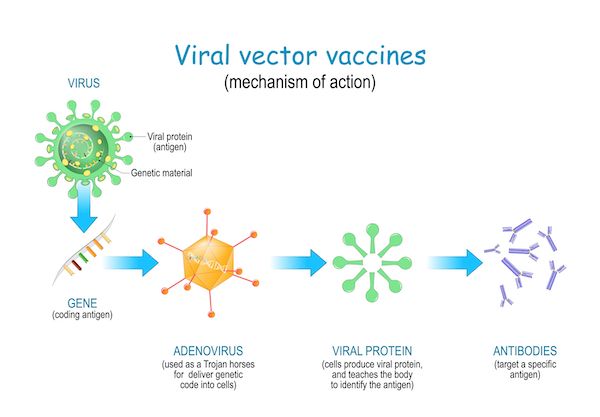
With this vector, the spike gene is transported inside the host muscle and other cells, which are “instructed” to synthetise the Spike protein on their surface. As a response to this foreign protein, the body will mount an immune response with the production of specific antibodies.
The concept of a vaccine is to mimic the viral behaviour without the detrimental effects of the virus itself. During the actual COVID-19 infection, this outer viral Spike protein binds to human cell receptors to infect the cell. The majority of these COVID-19 vaccines has been designed to deliver either the protein, the DNA (AstraZeneca) or RNA (Pfizer, Moderna) encoding the Spike protein in order for the vaccinated person to generate antibodies and thus prevent severe symptoms or death upon infection with the real virus. The AstraZeneca vaccine has demonstrated a 63% efficacy in reducing severe symptoms and death.
The most common side effects associated with COVID-19 Vaccine AstraZeneca are mild and comparable to those arising from a flu vaccine. They usually improve within a few days after vaccination, however, thrombosis and thrombocytopaenia have been reported in some cases; and an extremely low percentage have died after the vaccine administration.
What is a COVID-19 induced thrombosis? Is this different from other types of thrombosis?
The thrombosis as we know.
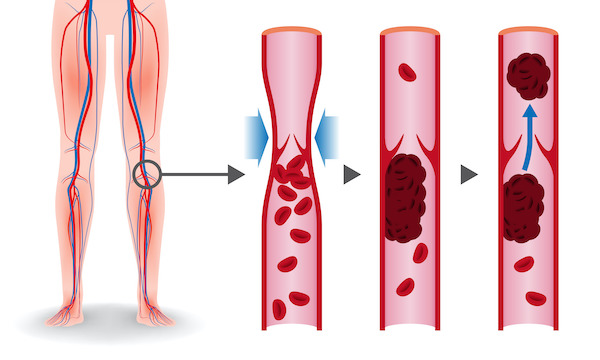
A blood clot is known as a thrombus. Superficial thrombi can occur in a skin cut, but usually a thrombosis occurs when a blood clot forms in a vein or an artery. Arterial and venous thrombosis can cause serious problems. Arterial thrombosis affects people with atherosclerosis or fatty deposits in the wall of the arteries and can cause a heart attack or a stroke.
Deep vein thrombosis (DVT) occurs when a blood clot forms in one or more of the deep veins in the body, usually in the legs. This can be the consequence of certain medical conditions that affect clotting. A blood clot in the legs can occur after a prolonged lack of movement, after surgery or trauma, when traveling a long distance, or when on bed rest. DVTs can break loose, travel through the bloodstream and get stuck in the lungs, blocking blood flow (pulmonary embolism). Risk factors for thrombosis are:
- Recent surgery
- Smoking
- Immobilisation for long periods
- Overweight or obesity
- Pregnancy
- Cancer
- Hormone therapy (including oral contraceptives)
- Blood disorders
- Dehydration
- Previous DVT
Which are the possible causes for increased thromboses?
The causes leading to increased thrombotic events after vaccination may be due to a number of factors:
Tissue Plasminogen Activator (TPA) – Beside the DNA encoding the spike protein, the COVID-19 vaccine contains a signal sequence for another protein, TPA, to increase immunogenicity in the host. TPA can interfere with the chain of molecular events of the coagulation process, ending with the formation of blood clots.
Adenovirus particles – Particles contained in the adenovirus, used as a vector to inoculate the Spike protein gene, can bind to a platelet factor resulting in the activation and aggregation of platelets.
Systemic inflammation – the incidence of blood clots is not only associated with the AstraZeneca vaccine but has also been observed in patients infected with COVID-19. We have often heard of the cytokine storm, or Systemic Inflammatory Response Syndrome (SIRS). SIRS involves an exaggerated systemic production of the inflammatory proteins named cytokines, which can themselves activate the coagulation process mediated by platelets and endothelial cells lining veins and arteries.
Antibody production – the immune response following the vaccination consists in the synthesis of anti-spike protein antibodies. Antibodies can also activate the coagulation process.
Disseminated Intravascular coagulation (DIC) – The combination of these factors begins an auto-amplification loop, which sustains the formation of blood clots not only in restricted vascular regions but also throughout the body leading to a condition referred to as DIC. Despite forming blood clots at multiple locations, this condition can cause profound and unstoppable bleeding, since all coagulation factors are consumed. A patient with such a condition is at high risk of dying.
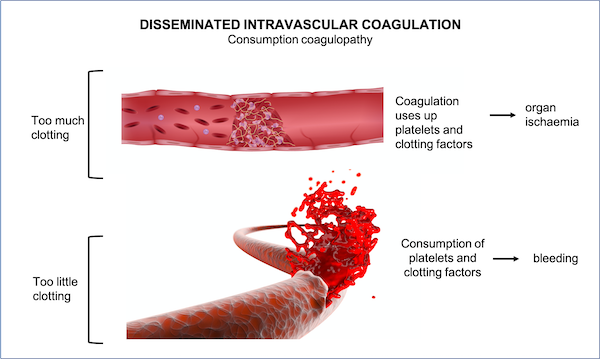
The COVID-19 vaccine paradox: Co-occurrence of thrombotic events with thrombocytopaenia
The thrombotic events resulting after COVID-19 infection and vaccine are very different from the most frequent forms of the condition. They are intrinsically mediated by the activation of the immune system.
The manifestation of vascular disorders after COVID-19 vaccine is puzzling due to the simultaneous increase of platelet forming blood clots with the striking reduction in the number of platelets in blood circulation. This condition is defined thrombocytopaenia.
VIPIT stands for Vaccine Induced Prothrombotic Immune Thrombocytopenia, a term implying clotting of thrombocytes (platelet cells) in the blood vessels in various parts of the body after receiving the COVID-19 vaccine.
A similar phenomenon to this paradoxical effect has been observed in 1 to 3 % of patients (without COVID-19) receiving heparin, as blood thinner used to prevent thrombosis formation. 4 to 10 days following heparin therapy, these patients had a low count of platelets in their blood and yet continued to form life-threatening clots as well as bleedings. This complication has been defined as heparin-induced thrombocytopaenia. The rapid fall in the number of circulating platelets seems to be driven by “consumption” caused by excessive formation of thrombi.
What is the incidence of COVID-19 vaccine thromboses?
Analysis of data from European countries confirms some association of the adenovirus-based vaccine and thromboses. About 170 cases of clots from the European reporting agencies and 19 deaths amongst the AstraZeneca vaccine recipients in the U.K. have been described so far. While overall that brings the incidence of VIPIT to about 1 in 500,000, those numbers vary by country. For example, reports from the Norwegian literature put the incidence of VIPIT at 1 in 25,000 vaccine recipients under the age of 65.
When and where do these adverse blood clotting events occur after the vaccine shot?
Within 14 days after vaccination a few patients developed thromboses within the mesenteric vein located in the abdomen as well as in the cerebral venous sinus. These thrombotic complications were mostly observed in women under the age of 55, following the AstraZeneca vaccine. Because the number of cases with such complications exceeded the expected incidence assessed by the producers, the EMA as well as several other governmental health ministries decided to temporarily halt the utilisation of the AstraZeneca vaccine to determine the correlation of the vaccine with these thrombotic events.
Although an association has been postulated, the causality between the vaccine and the thrombotic complications remains elusive. Due to the young age of the group affected by these complications, upon resumption of AstraZeneca vaccination, the vaccine has been recommended to people over 50 years of age.

Thrombocytopaenia and thrombosis also occur in COVID-19 disease
The incidence of thrombocytopaenia in patients with COVID-19 has been reported since the beginning of the pandemic and varies across studies. Mild thrombocytopaenia has been observed in 30% of these patients, with a higher frequency relative to the gravity of the symptoms in critically ill patients (57.7%) compared with non-severe disease (31.6%). COVID-19 also led to strokes in around 1.6% of people treated in the ICU.
Immune thrombocytopaenia seems to occur in 20% of cases 3 weeks after onset of COVID-19 symptoms, with other studies describing it after clinical recovery. The proposed mechanisms of thrombocytopaenia with COVID-19 involve inhibition of platelet synthesis due to:
- Direct infection of the bone marrow cells or platelets by the virus
- Virus-mediated liver damage leading to decreased thrombopoietin production
- Pulmonary endothelial damage followed by platelet aggregation in the lungs, subsequent formation of microthrombi, and platelet consumption
- Finally, the destruction of platelets by the immune system.
What is cerebral venous sinus thrombosis (CVST)?
Cerebral venous sinus thrombosis (CVST) is a blood clot that forms in a region of the brain called the venous sinuses. The venous sinuses help to drain blood from the brain into the circulation. The clot prevents blood from draining out of the brain. Stagnating blood can leak into brain tissue and cause a haemorrhage or bleeding. The clot and bleeding cause a stroke. The symptoms of a CVST are:
- Headache
- Blurred vision
- Fainting or loss of consciousness
- Loss of control over movement in part of the body
- Seizures
- Coma
Blood clotting is extremely rare. Benefits outweigh risks
Investigations by EU and UK regulators into reports of unusual blood clots after receiving the AstraZeneca vaccine have concluded that these are a “possible” and “extremely rare” side effect. Neither agency established a causal relation.
The EMA’s investigating committee reviewed 62 cases of cerebral venous sinus thrombosis and 24 cases of splanchnic vein (abdominal region) thrombosis (reported on 22 March 2021), 18 of which were fatal. At such time, around 25 million people in the EU and UK had already received the AstraZeneca vaccine.
Among more than 20 million people who have been vaccinated with the AstraZeneca vaccine in the UK, 79 cases of rare blood clots with low platelets have been reported, as well as 19 deaths.
This equates to around one case per 250 000 people vaccinated – 0.0004% – and one death in a million.
Independent of COVID-19, in the wider population, about 100 000 people usually develop blood clots every month in the EU, and 3000 a month in the UK.
Putting the risk in context
1 in 250 000 (0.0004%) people with the AstraZeneca vaccine will develop blood clots with low platelets
1 in 2000 (0.05%) women each year will develop a blood clot from taking the combined oral contraceptive pill
1 in 1000 (0.1%) people a year will develop a blood clot (deep vein thrombosis) from air travel.
In other words, many more women get a blood clot by taking the oral contraceptive pill and many more people will get a blood clot due to travelling.
The EMA has released a statement to provide a sensible perspective on the incidence of thrombotic complications in patients receiving the COVID-19 vaccine in relation to the general population.
Sabine Straus, MD, chair of the EMA’s Pharmacovigilance Risk Assessment Committee (PRAC), stated: ” Our conclusion is that benefit of the AstraZeneca vaccine is far greater than the risks. There is also no evidence of a quality issue with a particular batch of vaccine.”
Dr Straus added: “Our conclusion is that these clotting disorders are very rare side effects of the vaccine. This conclusion is based on the distinct clinical pattern, immunological findings, and observation of more reported cases than would have been expected.”